Customized Deviation Management Systems by Zenkins
In highly regulated industries such as pharmaceuticals, biotechnology, and manufacturing, managing deviations effectively is crucial to maintaining quality and compliance. Deviations occur when there is a departure from standard operating procedures (SOPs) or expected results, and they must be addressed promptly to ensure product integrity and regulatory adherence. At Zenkins, we specialize in developing customized Deviation Management Systems tailored to meet the unique needs of your organization. Our solutions streamline the deviation management process, enhance accountability, and ensure compliance with industry standards.
Screens
What is a Deviation Management System?
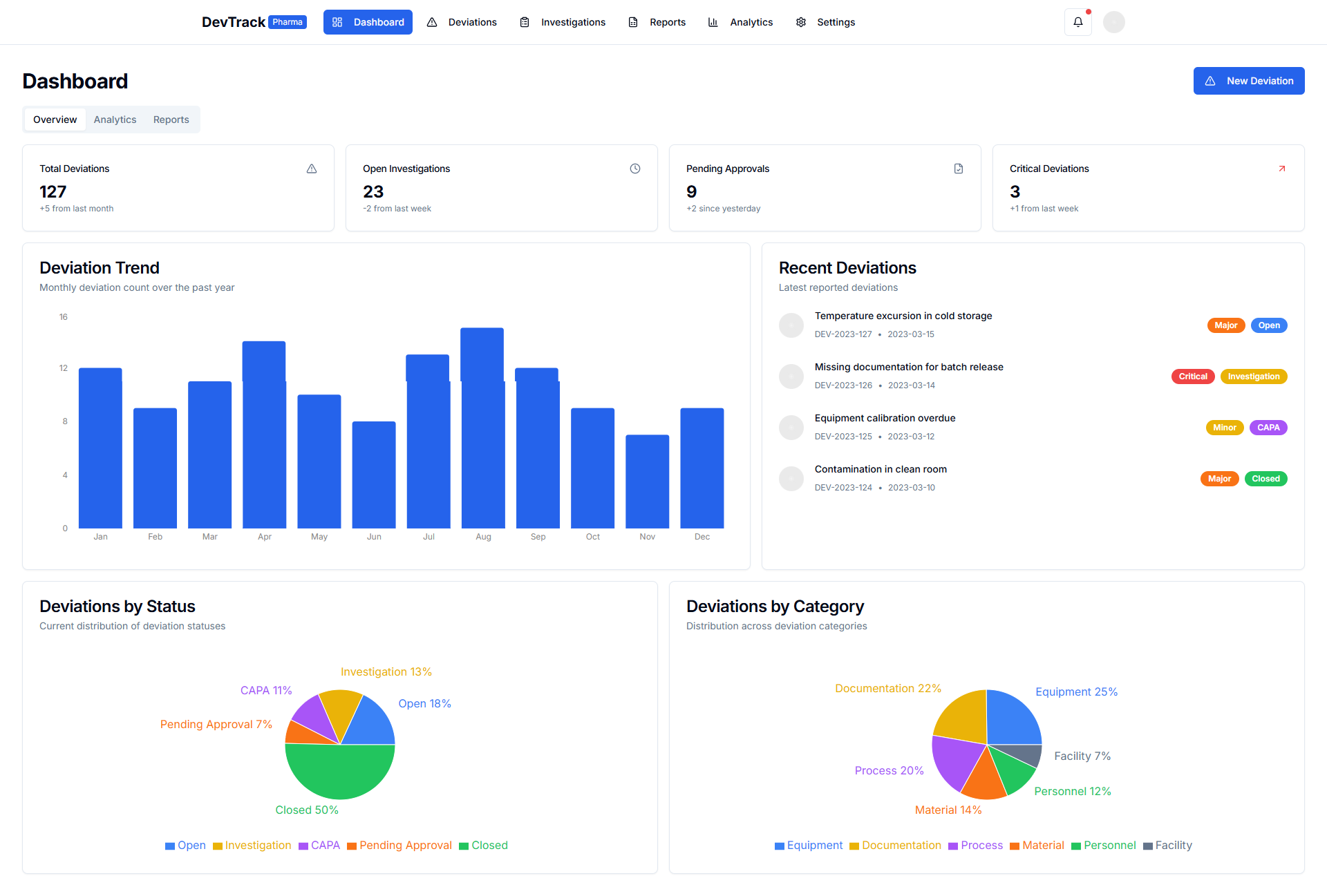
A Deviation Management System is a comprehensive tool designed to document, track, investigate, and resolve deviations from standard procedures or expected results. It ensures that all deviations are properly managed and documented, corrective actions are implemented, and compliance with regulatory requirements is maintained. By centralizing and automating the deviation management process, organizations can improve efficiency, reduce risks, and enhance product quality.
Key Benefits of Implementing a Deviation Management System
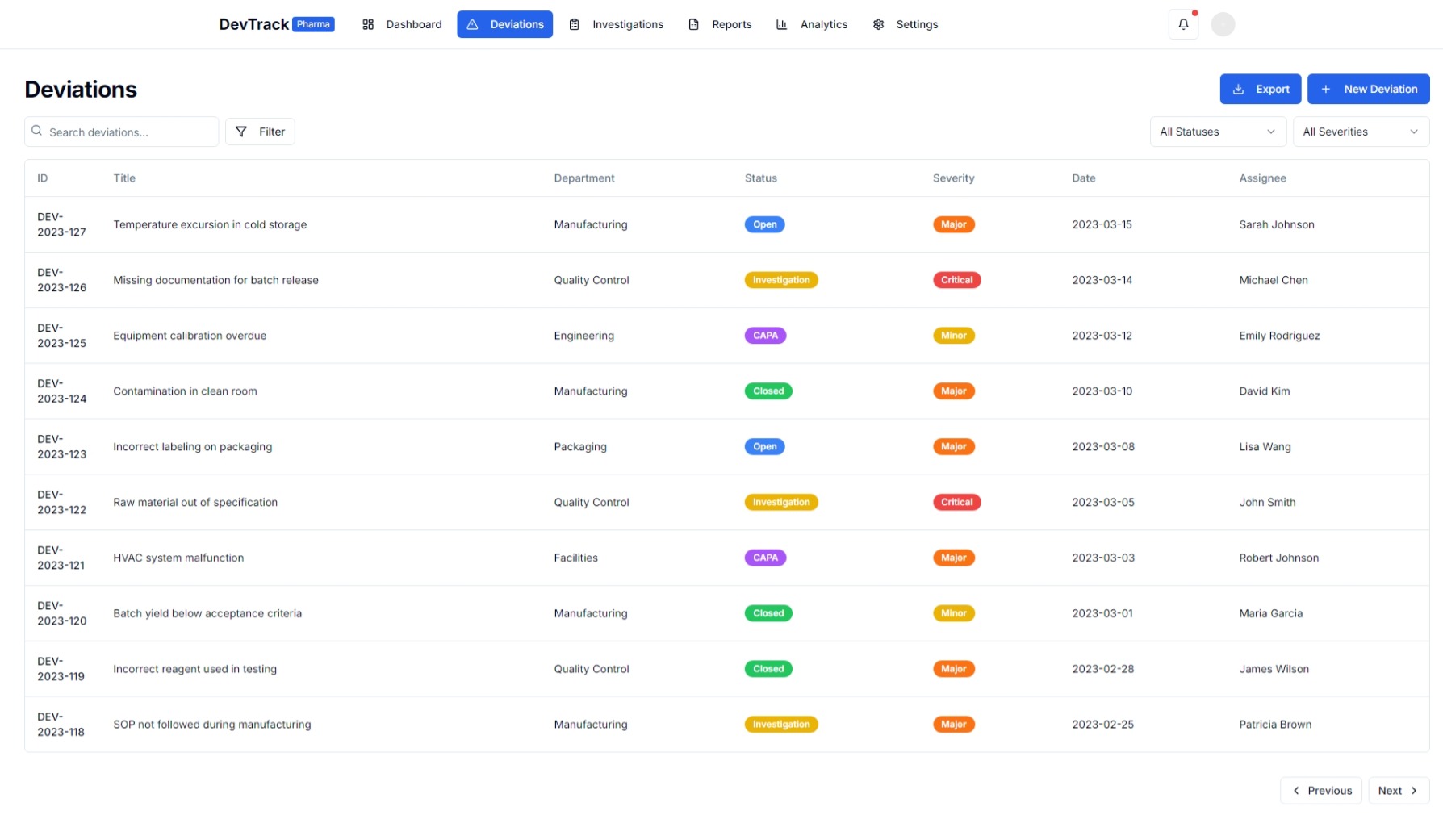
1. Centralized Documentation and Tracking
- Unified Repository: A single, centralized repository for all deviation-related information, including initial reports, investigation details, and corrective actions.
- Traceability: Ensures complete traceability of deviations from detection to resolution.
- Audit Trails: Maintains detailed audit trails to support regulatory inspections and audits.
2. Streamlined Workflow Management
- Automated Workflows: Automates the deviation management workflow from reporting and investigation to corrective action and closure.
- Customizable Processes: Tailors workflows to match your organization’s specific procedures and requirements.
- Real-Time Notifications: Sends alerts for pending tasks and deadlines, ensuring timely completion of activities.
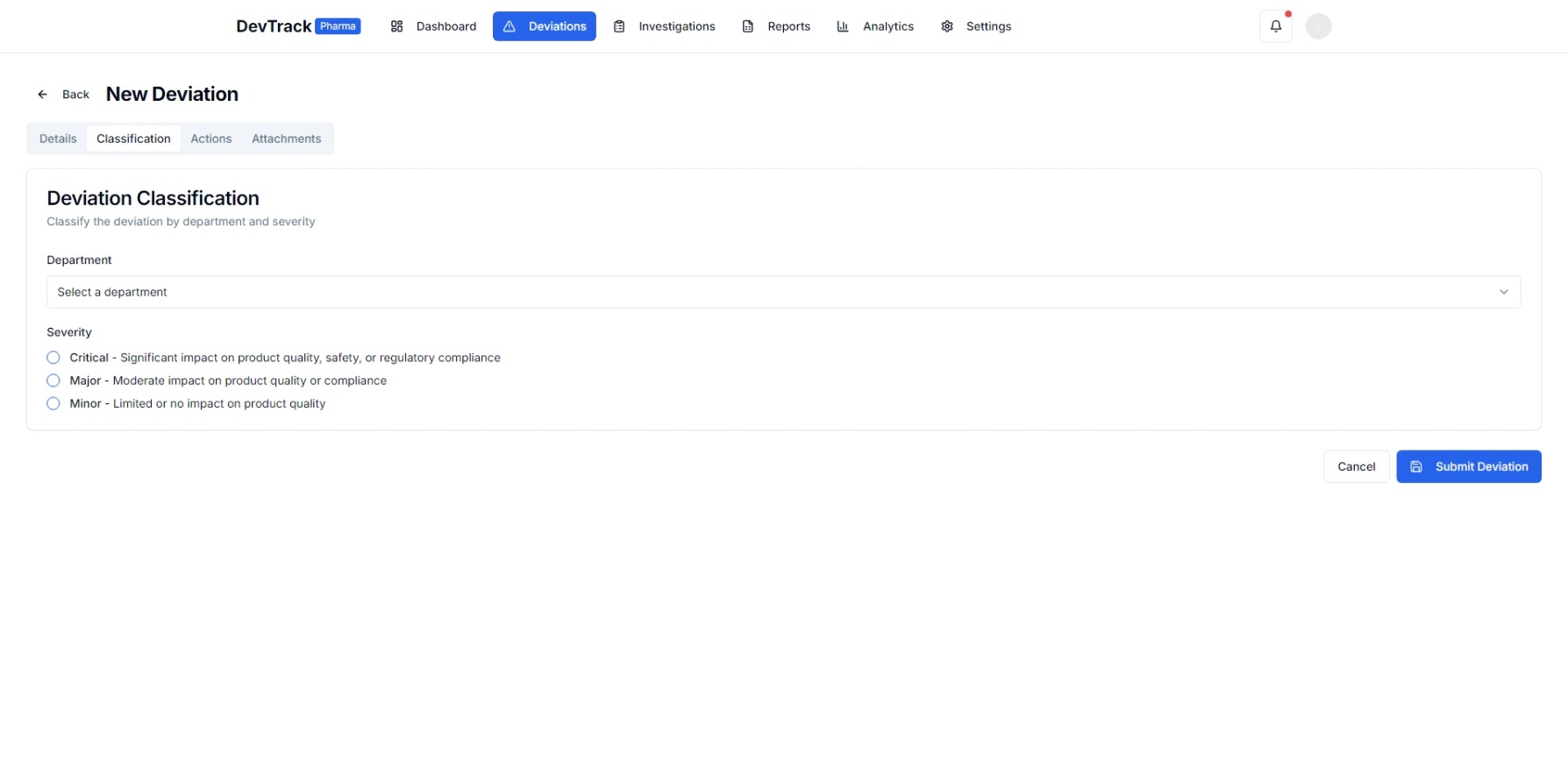
3. Enhanced Compliance and Quality Control
- Regulatory Compliance: Ensures adherence to industry standards and regulatory requirements throughout the deviation management process.
- Quality Assurance: Integrates quality management processes to monitor and improve product quality.
- Standardized Procedures: Enforces standardized procedures for deviation reporting, investigation, and resolution.
4. Improved Root Cause Analysis and Corrective Actions
- Root Cause Analysis: Facilitates thorough root cause analysis to identify the underlying causes of deviations.
- Corrective and Preventive Actions (CAPA): Implements effective corrective and preventive actions to prevent recurrence of deviations.
- Continuous Improvement: Promotes continuous improvement through ongoing monitoring and analysis of deviation trends.
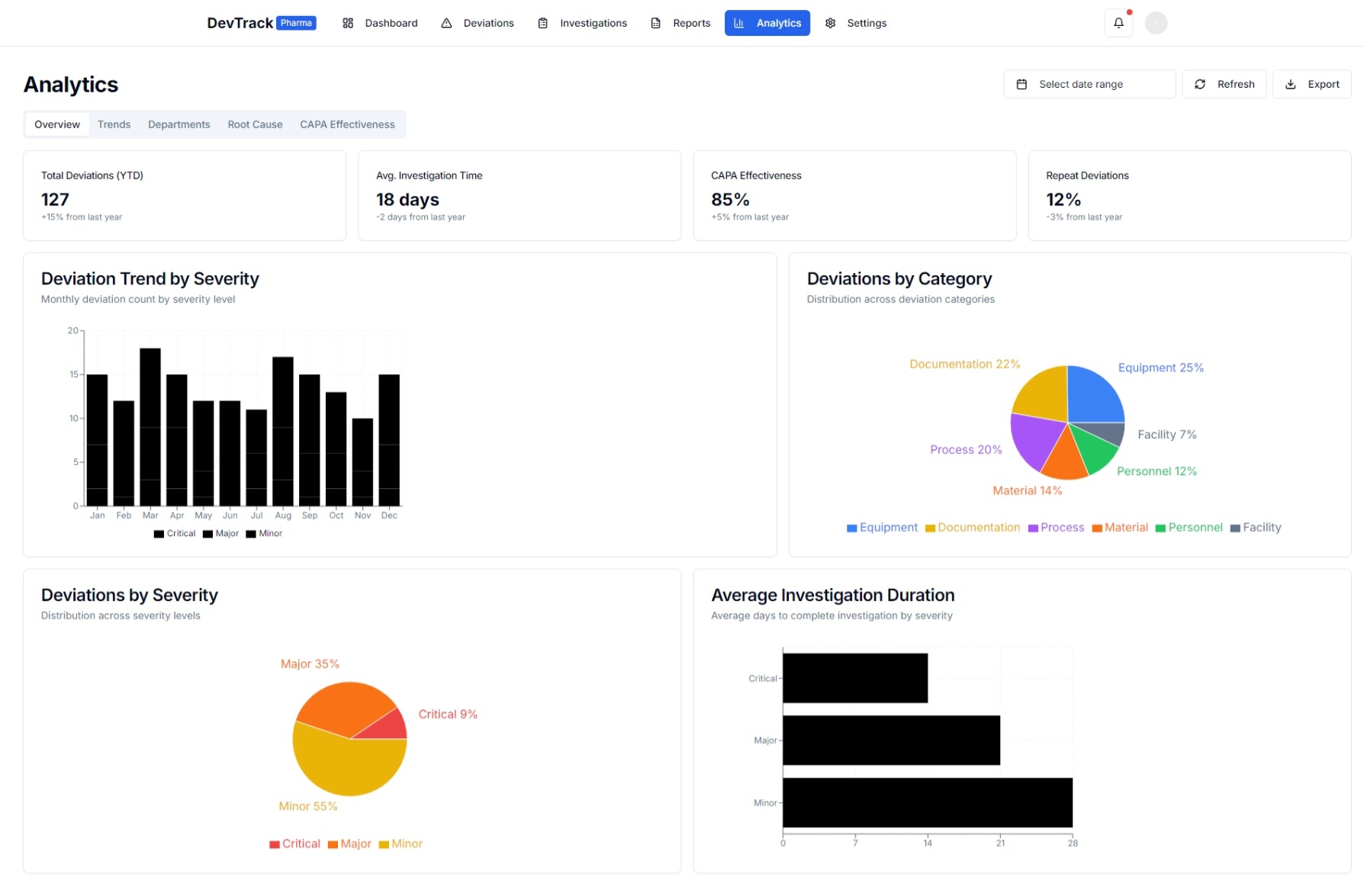
5. Advanced Reporting and Analytics
- Comprehensive Reports: Generates detailed reports to monitor deviation status, trends, and key metrics.
- Customizable Dashboards: Provides real-time insights with customizable dashboards.
- Data-Driven Decisions: Utilizes advanced analytics to identify trends, opportunities, and areas for improvement.
6. Enhanced Accountability and Transparency
- Clear Roles and Responsibilities: Defines clear roles and responsibilities for deviation management tasks.
- Transparent Processes: Ensures transparency in the deviation management process, fostering a culture of accountability.
- Documented Approvals: Tracks and documents all approvals and decision-making processes.
Why Choose Zenkins for Your Deviation Management System?
1. Expertise in Custom Software Development At Zenkins, we bring years of experience in developing custom software solutions across various industries. Our team of skilled developers, designers, and project managers work collaboratively to deliver a Deviation Management System that aligns perfectly with your operational requirements and strategic goals.
2. Comprehensive Needs Assessment We start with a thorough assessment of your current processes and requirements. By understanding your specific needs, we design a Deviation Management System that integrates seamlessly with your existing workflows and addresses all your unique challenges.
3. Scalable and Flexible Solutions Our Deviation Management Systems are scalable and flexible, capable of growing with your business. Whether you need a simple deviation tracking tool or a comprehensive system with advanced features, we can deliver a solution that fits your needs.
4. Enhanced Security and Compliance Zenkins prioritizes data security and regulatory compliance. Our systems are built with robust security measures to protect sensitive information and ensure compliance with industry standards and regulations.
5. User-Friendly Interface We focus on creating intuitive and user-friendly interfaces that simplify the deviation management process. This ensures that all users, regardless of technical proficiency, can efficiently use the system.
Implementation and Support
1. Tailored Implementation Plan Our team works closely with you to develop a tailored implementation plan that minimizes disruption and ensures a smooth transition to the new system. We provide comprehensive training and support to ensure your team is fully equipped to use the system effectively.
2. Ongoing Support and Maintenance Zenkins offers ongoing support and maintenance services to keep your Deviation Management System running smoothly. Our support team is available to assist with any issues, updates, or enhancements needed to keep your system up-to-date and fully functional.
3. Continuous Improvement We believe in continuous improvement and work closely with our clients to gather feedback and make necessary enhancements to the system. This ensures that your Deviation Management System remains effective and evolves with your business needs.
Key Features of Zenkins’ Deviation Management System
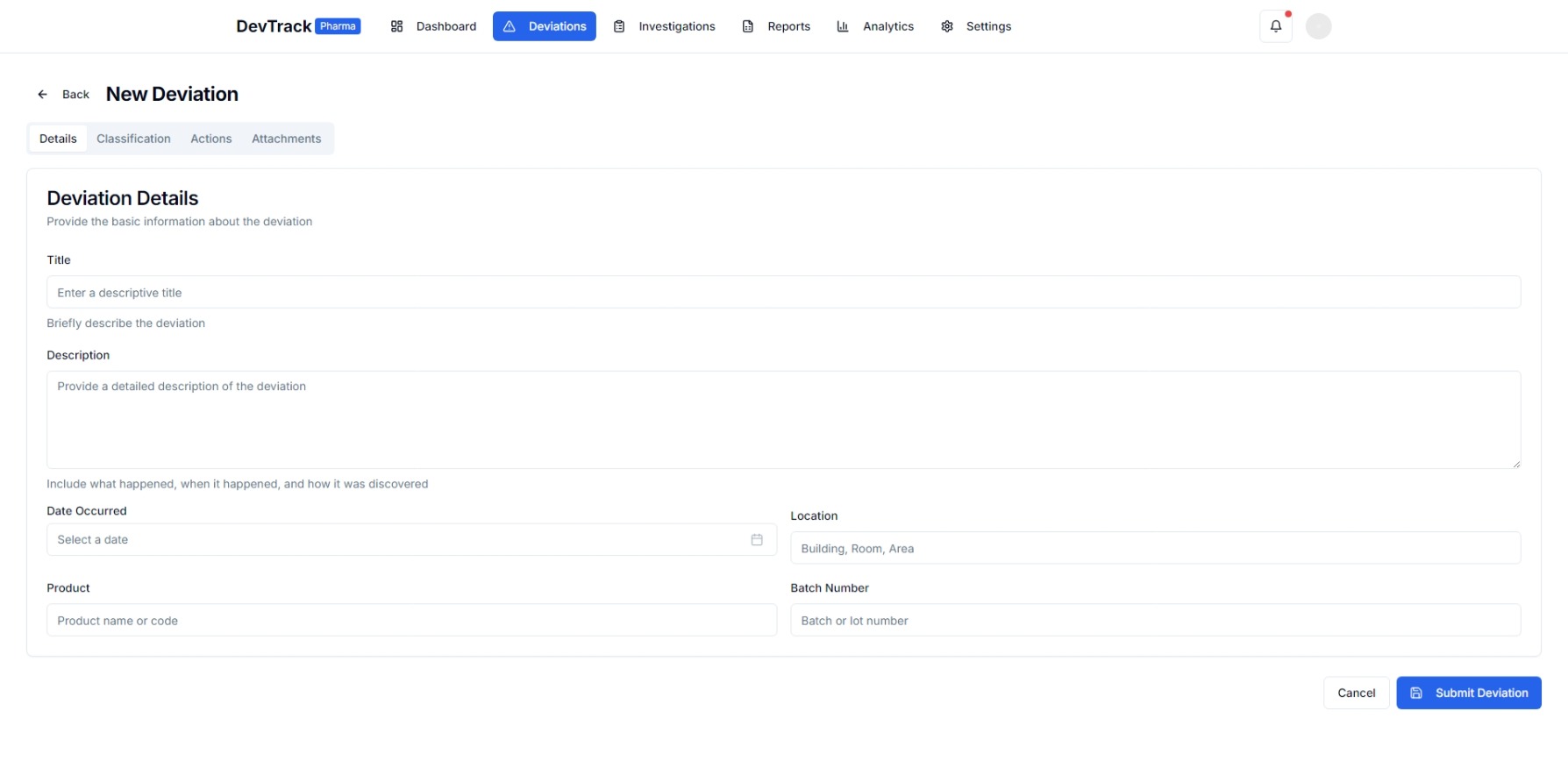
1. Deviation Reporting and Documentation
- Incident Reporting: Easy-to-use interfaces for reporting deviations with detailed incident descriptions.
- Automated Documentation: Automatically generates and stores all necessary documentation for each deviation.
2. Investigation and Root Cause Analysis
- Investigation Tools: Provides tools for thorough investigation and root cause analysis.
- Collaborative Investigations: Enables cross-functional teams to collaborate on investigations and share insights.
3. Corrective and Preventive Actions (CAPA)
- Action Plans: Develops and tracks corrective and preventive action plans.
- Effectiveness Checks: Monitors the effectiveness of implemented actions to ensure they address the root causes.
4. Regulatory Compliance and Audit Readiness
- Compliance Checklists: Built-in checklists to ensure adherence to regulatory standards.
- Audit Support: Generates reports and documentation to support regulatory audits and inspections.
5. Reporting and Analytics
- Real-Time Reporting: Provides real-time reports on deviation status, trends, and key performance indicators (KPIs).
- Customizable Dashboards: Allows customization of dashboards to focus on the metrics that matter most to your organization.
6. Integration Capabilities
- System Integration: Seamlessly integrates with existing quality management systems (QMS), enterprise resource planning (ERP) systems, and other business applications.
- Data Import/Export: Supports data import and export to facilitate information sharing and reporting.
Why Zenkins?
Choosing Zenkins for your Deviation Management System development ensures you receive a customized, secure, and efficient solution that meets all your operational and compliance needs. Our commitment to quality, flexibility, and customer satisfaction sets us apart as a trusted partner in your journey towards enhanced quality management and regulatory compliance.
Contact us today to learn more about how we can help you develop a customized Deviation Management System tailored to your specific requirements. Experience the Zenkins difference and elevate your deviation management processes to new heights.